Digitalization in the Pharmaceutical Industry
What is the current status of digitalization in the pharmaceutical industry? Where does it already help and what other opportunities do software solutions offer?

13. July 2023
It is indisputable - we live in a digital age. Digitalization is firmly embedded in almost every industry. But what about the highly regulated pharmaceutical industry? Is the digital route even possible? In this article, I’ll take a look at the opportunities and challenges of digitalization in the highly regulated environment of the pharmaceutical industry.
Digitalization in the pharmaceutical industry: The current status.
It should come as no surprise that digitization is impacting the pharmaceutical industry. With new digital technology, companies can optimize the entire life cycle of pharmaceutical products. For example, pharmaceutical companies are now able to implement digital data streams that span the entire value chain. This is referred to as the “digital thread," and it links various interrelationships within the company, such as the cooperation between humans and machines in the form of networked production and the embedding of machine learning. The result is increased transparency throughout the company.
It is clear that digital solutions are having a far-reaching impact on the everyday life of the pharmaceutical industry, and in some cases, are already changing it. And yet, many companies are still hesitating due to the human instinct to carry on as before, or the concern that they will not be able to comply completely with the regulations. By not keeping up with digital developments, they risk being left behind, and then only being able to meet current requirements by investing significantly in time and effort.
A concrete guide to digital success in the pharmaceutical industry
We will show you how to identify the processes in your pharmaceutical company that you should digitize and give you practical recommendations for action.

Digitalization in the pharmaceutical industry: How it helps
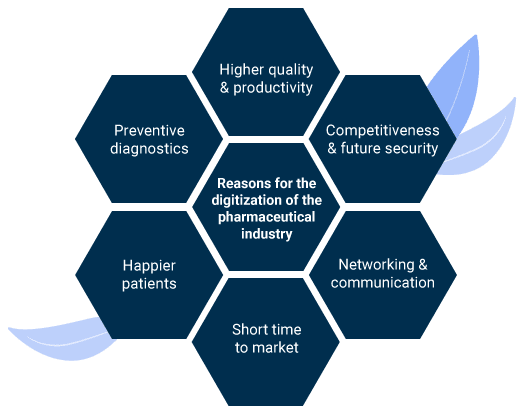
Good reasons for the digitalization of pharmaceutical companies
Companies that want to be successful in the long term cannot avoid digital solutions. Most pharmaceutical companies have already taken the first digital steps or converted relevant processes. Regardless of the company's level of digitalization, here are some good reasons for following this path:
-
Networking and communication
Digital solutions simplify the exchange of information and documents, such as patient records - both internally and externally, as digital records can be accessed independent of location, and even on the go. Providing a more efficient exchange of information between employees, partners, physicians, pharmacists, and patients. Digital technologies make it easier for employees and partners to communicate with each other, collaboration is improved, and sharing information is made easier. The result is obvious: faster decision-making processes and greater efficiency.
-
Competitive and future-proof
New, innovative companies with digital capabilities are entering the market as competitors. To remain ahead of the game, you have to move with the times. With digital solutions, you can deal with market changes more agilely. Pharmaceutical companies that take advantage of digitization are usually better positioned to compete with new and existing competitors.
-
Increased productivity
Digital processes save time. This is good news for employees, who can concentrate on more important tasks, and also increases the output of the entire company.
-
Shorter market launch period
In research and development, digitalization presents a significant advantage for pharmaceutical companies. The use of Big Data and artificial intelligence ensures that even large amounts of data can be evaluated quickly, providing swift analysis results. With computer-aided modeling and simulation, the efficacy and safety of drugs can be established quickly. This leads to increased innovation, faster development of new products, and a shorter go-to-market period.
-
Increased patient satisfaction
The use of digital patient records, personalized medicine, and virtual consultations results in improved and more targeted communication. Patients often feel better understood and are more satisfied with their care.
-
Higher quality
Production is improved due to data analysis, monitoring systems, and digital quality assurance tools. Faulty or defective products are detected earlier and, in the best case, direct intervention in the production process is possible. This increases the quality of products.
-
Preventive diagnostics
Digital tools provide physicians with diagnostic support. Artificial intelligence is at the forefront of this field, enabling novel, detailed imaging, and evaluation.
The transition to Pharma 5.0. What's new?
Will Pharma 4.0 soon be old news? The future points in the direction of Pharma 5.0, which encompasses the digital transformation of the pharmaceutical industry. The focus is on drug manufacturing in the clean room, the distribution process, and a change of usage. The goal is more efficient production, higher quality, and a better relationship between patients and pharmaceutical companies. To achieve this, Pharma 5.0 relies on Big Data and artificial intelligence, and benefits from cloud computing. Augmented reality and the Internet of Things (IoT) play a key role in Pharma 5.0.
Pharma 5.0 typically involves these innovations:
-
Personalized medicine
Data analytics and artificial intelligence generate more targeted treatment plans and tailor drug efficacy to individual patient needs.
-
Digitalized drug production
The Internet of Things, artificial intelligence, and cloud computing digitally support drug production. Pharma 5.0 uses all these technologies in a targeted way to gain even more specific insights. In addition, the focus is on process optimization and improved patient care.
-
Tapping into new business models
Companies are finding it easier to tap into new business models by using blockchain, which is a database that chronologically appends more and more data to an initial block of data, making each step traceable, and smart contracts, which are computer protocols for mapping contracts. This increases transparency in the pharmaceutical industry and improves cooperation.
Pharma 5.0 also aims to better connect various players in the healthcare system and optimize the exchange of information. This also enables cross-departmental teams to tackle complex tasks more easily and in a centrally controlled manner.
-
Ethics and sustainability
The topics of Pharma 5.0 raise questions about ethics and sustainable practices. Responsible, long-term production of medicines is the goal. Ecological, social, and economic aspects are taken into account, and privacy and data protection requirements are applied - particularly with regard to the handling of sensitive health data.
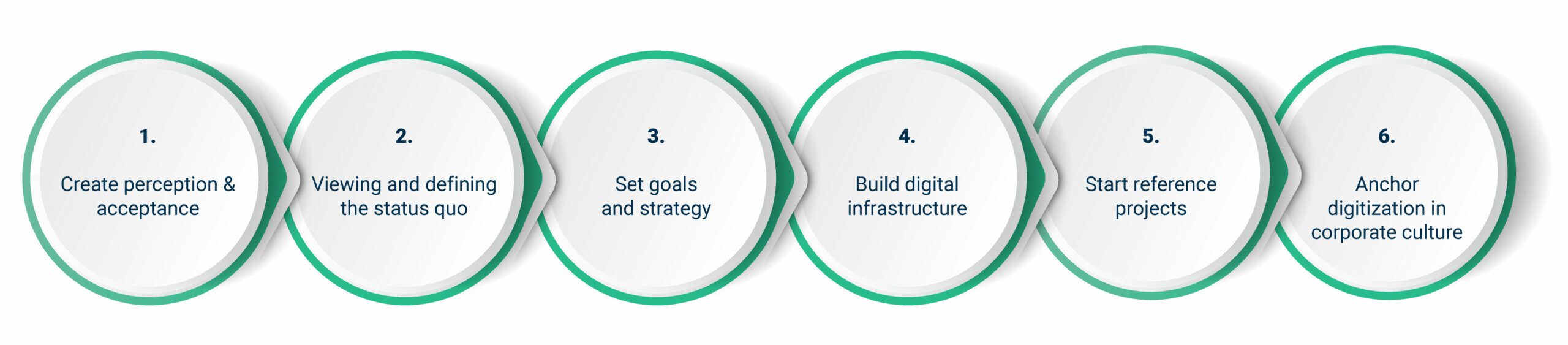
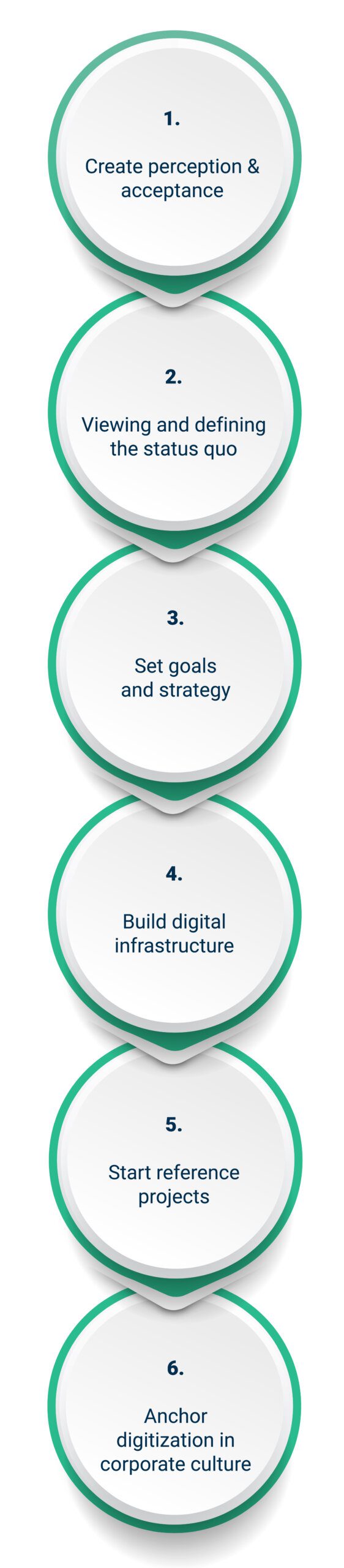
Digitalizing a pharmaceutical company – how does that actually work?
Do you just start digitalizing? That’s not a great idea. Organizations need a well-designed digitalization strategy to complete the process in a targeted manner, and with long-term success. This includes several steps:
The challenges of digitization for pharmaceutical companies
Digitization offers many opportunities. At the same time, however, it also presents companies with challenges. Typical hurdles faced by the pharmaceutical industry include the following:
-
Data silos
Companies that strive toward automating processes often start from highly manual processes. File here, document there. The problem is that without continuous digital storage, data silos are created. To take full advantage of the benefits of digitalization, all data must first be collected and processed in a uniform manner.
-
Inconsistent approach
The defined process must be followed precisely for digitalization to succeed, and the benefits fully realized in the long term. If management or key functions pull back or refuse to use digital tools, gaps are created. The same effect occurs if the defined strategy is not implemented.
-
Resistance to change
"But we've always done it that way." This is a typical sentence that comes up repeatedly during the digitalization process - and this way of thinking is a hindrance. Only those who are open to change can implement it successfully.
-
Data security and regulations
Digitization is closely linked to cloud solutions and the concern for data security. This is an important topic that should definitely be taken into account - especially in the regulated environment of the pharmaceutical industry. Because not all software meets the requirements of data security and the requirements that authorities specify. The highly regulated production of pharmaceuticals in the clean room, which is subject to GMP regulations, must be prepared for this.
-
Personnel and resources
The labor market is in a state of flux. Existing job descriptions are being joined by new ones. Employees need to learn new skills and move with the times. Another challenge is the shortage of skilled workers. In many areas of operation, there are not enough resources, and the work that needs to be done cannot be done.
Which Software can help?
The solution that pharmaceutical companies can use to meet the challenges is software. However, right from the start, you should ensure that the solution meets your strict requirements and is future-proof.
An ERP system such as Microsoft Dynamics 365 Business Central is helpful, because it is tried and tested, specially designed for the requirements of medium-sized businesses, and is constantly being optimized. Is that enough for the pharmaceutical industry? The requirements in the pharmaceutical environment go beyond standard functions. The solution is a special ERP industry software that extends the Microsoft ERP - like YAVEON ProBatch. Our solution includes the features that turn a good standard ERP into a specialist for your own industry.
For example:
Reliable and regular quality controls that are both regulatory and compliance compliant.
Full responsiveness thanks to batch management and traceability
Certainty in any audit, as the appropriate ERP system includes the necessary functions to support the regulatory requirements of the pharmaceutical industry. This includes, for example, batch tracing and the documentation of GMP requirements.
Shelf life and expiration controls for less waste and maximum efficiency
Overview of inventories thanks to warehouse and logistics management, by optimizing the management and storage of inventories.
Central database, as the various business processes and work areas are integrated with each other and handled centrally.
The right ERP for the pharmaceutical industry
Optimize your processes in the pharmaceutical industry with our ERP industry solution. Your key to successful digitalization and optimized operational processes.
Conclusion
Digitalization has arrived in the pharmaceutical industry and is here to stay. Companies benefit from looking at the options and finding efficient ways to implement them. Those who don't will fall by the wayside in the long run. It is important to rely on software that meets the strict requirements and is on the market in the long term.
Take advantage of this opportunity and become a digital trailblazer!






